
Chemistry Paper 2 (233/2)
1 The flow chart below shows some of the processes involved in large scale production of sulphuric (VI) acid. Use it to answer the questions that follow.

(a) Describe how oxygen is obtained from air on a large scale. (3 marks)
(b) (i) Name substance A. (1 mark)
(ii) Write an equation for the process that takes place in the absorption chamber. (1 mark)
(c) Vanadium (V) oxide is a commonly used catalyst in the contact process.
(i) Name another catalyst which can be used for this process. (1 mark)
(ii) Give two reasons why vanadium (V) oxide is the commonly used catalyst. (2 marks)
(d) State and explain the observations made when concentrated sulphuric (VI) acid is added to crystals of copper (II) sulphate in a beaker. (2 marks)
(e) The reaction of concentrated sulphuric (V1) acid with sodium chloride produces hydrogen chloride gas. State the property of concentrated sulphuric (Vi) acid illustrated in this reaction. (1 mark)
(f) Name four uses of sulphuric (VI) acid. (2 marks)
2 The set-up below was used by a student to investigate the products formed when aqueous copper (ll) chloride was electrolysed using carbon electrodes.

(a) (i) Write the equation for the reaction that takes place at the cathode. (1 mark) (ii) Name and describe a chemical test for the product initially formed at the anode when a highly concentrated solution of copper (ll) chloride is electrolysed. (3 marks)
(iii) How would the mass of the anode change if the carbon anode was replaced with copper metal? Explain. (2 marks)
(b) 0.6 g of metal B Were deposited when a current of O.45A was passed through an electrolyte for 72 minutes. Determine the charge on the ion of metal B. (Relative atomic mass of B = 59, 1 Faraday = 96 500 coulombs) (3 marks)
(c) The electrode potentials for cadmium and zinc are given below:

Explain why it is not advisable to store a solution of cadmium nitrate in a container made of zinc. (2 arks)
3 (a) Ethanol can be manufactured from ethene and steam as shown in the equation below:
![]()
Temperature and pressure will affect the position of equilibrium of the above reaction. Name the other factor that will affect the position of equilibrium of the above reaction. (1 mark)
(b) The data in the table below was recorded when one mole of ethene was reacted with excess steam. The amount of ethanol in the equilibrium mixture was recorded under different conditions of temperature and pressure. Use the data to answer the questions that follow.

(i) State whether the reaction between ethene and steam is exothermic or endothermic. Explain your answer. (3 marks) .
(ii) State and explain one advantage and one disadvantage of using extremely high pressure in this reaction.
I. Advantage (2 marks)
II. Disadvantage (2 marks)
(c) In an experiment to determine the rate of reaction between calcium carbonate and dilute hydrochloric acid, 2g of calcium carbonate were reacted with excess 2 M hydrochloric acid. The volume of carbon (IV) oxide evolved was recorded at regular intervals of one minute for six minutes. The results are shown in the table below.

(i) Plot a graph of time in minutes on the horizontal axis against volume of carbon (IV) oxide on the vertical axis. (3 marks)
(ii) Determine the rate of reaction at 4 minutes. (2 marks)
4 (a) When excess calcium metal was added to 50 cm3 of 2 M aqueous copper (II) nitrate in a beaker, a brown solid and bubbles of gas were observed.
(i) Write two equations for the reactions which occurred in the beaker. (2 marks)
(ii) Explain why it is not advisable to use sodium metal for this reaction. (2 marks)
(b) Calculate the mass of calcium metal which reacted with copper (II) nitrate solution. (Relative atomic mass of Ca = 40) (2 marks)
(c) The resulting mixture in (a) above was filtered and aqueous sodium hydroxide added to the filtrate dropwise until in excess. What observations were made?(1 mark)
(d) (i) Starting with calcium oxide, describe how a solid sample of calcium carbonate can be prepared. (3 marks)
(ii) Name one use of calcium carbonate. (1 mark)
5. (a) Other than their location in the atom, name two other differences between an electron and a proton. (2 marks)
(b) The table below gives the number of electrons, protons and neutrons in particles A, B,C,D, E,F and G.
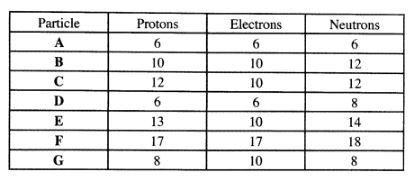
(i) Which particle is likely to be a halogen?(1 mark)
(ii) What is the mass number of E?(1 mark)
(iii) Write the formula of the compound formed when E combines with G.(1 mark)
(iv) Name the type of bond formed in (m) above.(1 mark)
(v) How does the radii of C and E compare? Give a reason.(2 marks)
(vi) Draw a dot (.) and cross (X) diagram for the compound formed between A and F.(1 mark)
(vii) Why would particle B not react with particle D?(1 mark)
6 (a) Study the flow chart below and answer the questions that follow.

(i) I What observation will be made in Step I?(1 mark)
II Describe a chemical test that can be carried out to show the identity of compound C.(2 marks)
(ii) Give the names of the following:(2 marks)
I E …………………………. II Substance D ……………… (iii) Give the formula of substance B.(1 mark)
(iv) Name the type of reaction that occurs in:(1 mark)
I Step (II) ……………………….
II Step (IV) ……………………….
(v) Give the reagent and conditions necessary for Step (2 marks)(VI). Reagent: …………………..
Conditions ……………… ..
(b) (i) Name the following structure.

(ii) Draw the structure of an isomer of pentene. (1 mark)
7 (a) What is meant by molar heat of combustion? (1 mark)
(b) State the Hess’s Law. (1 mark)
(c) Use the following standard enthalpies of combustion of graphite, hydrogen and enthalpy of formation of propane.

(i) Write the equation for the formation of propane. (1 mark)
(ii) Draw an energy cycle diagram that links the heat of formation of propane with its heat of combustion and the heats of combustion of graphite and hydrogen. (3 marks)
(iii) Calculate the standard heat of combustion of propane. (2 marks)
(d) Other than the enthalpy of combustion, state one factor which should be considered when choosing a fuel. (1 mark)
(e) The molar enthalpies of neutralization for dilute hydrochloric acid and dilute nitric (V)acid are -57.2k.j/mol while that of ethanoic acid is —55.2kJ/mol. Explain this observation. (2 marks)
