
Chemistry Paper 3 (233/3)
1 You are provided with:
– 1.6Og of solid A. a dibasic acid.
– Solution B containing 4.75g per litre of salt B.
– Aqueous sodium hydroxide. solution C.
– Phenolphthalein indicator.
You are required to prepare a solution of solid A and use it to determine the:-
– Concentration of sodium hydroxide. solution C
– React salt B with excess sodium hydroxide and then determine the relative molecular mass of salt B.
Procedure I
(a) Using a burette, place 25 .Ocm3 of solution B in each of two 250ml conical flasks.
Using a pipette and pipette filler, add 25.0cm3 of solution C to each of the two conical flasks. (The sodium hydroxide added is in excess). Label the conical flasks 1 and 2.
(b) Heat the contents of the first conical flask to boiling and then let the mixture boil for 5 minutes. Allow the mixture to cool.
(c) Repeat procedure (b) with the second conical flask.
While the mixtures are cooling, proceed with procedure II.
Procedure II
(a) Place all of solid A in 21 250 ml volumetric flask. Add about 150cm3 of distilled water, shake well to dissolve the solid and then add water to make up to the mark. Label this as solution A.
(b) Place solution A in a clean burette. Using a pipette and pipette filler, place 25 .0cm3 of solution C in a 250ml conical flask. Add 2 drops of phenolphthalein indicator and titrate with solution A. Record your results in Table 1. Repeat the titration two more times and complete the table.
Table 1
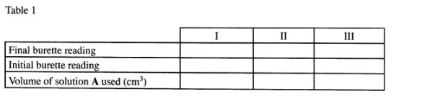
(4 marks)
Calculate the:-
(i) average volume of solution A used: (1/2 mark)
(ii) concentration in moles per litre of the dibasic acid in solution A; (2 marks) (Relative molecular mass of A is 126).
(iii) moles of the dibasic acid used; (1 mark)
(iv) moles of sodium hydroxide in 25 .0cm3 of solution C. (l mark)
(v) concentration of sodium hydroxide in moles per litre. (2 marks)
Procedure III
Add 2 drops of phenolpthalein indicator to the contents of the first conical flask prepared in procedure l and titrate with solution A. Record your results in Table 2. Repeat the procedure with the contents of the second conical flask and complete the table.
Table 2

(3 marks)
Calculate the:-
(i) average volume of solution A used; (1/2 mark)
(ii) moles of the dibasic acid used; (1 mark)
(iii) moles of sodium hydroxide that reacted with the dibasic acid. (1 mark)
(iv) moles of sodium hydroxide that reacted with 25 .0cm3 of salt B in solution B; (2 marks)
(v) Given that l mole of salt B reacts with 2 moles of sodium hydroxide, calculate the: l. number of moles of salt B in 25.0cm3 of solution B; (1 mark)
ll. concentration in moles per litre of salt B in solution B; (1 mark)
lll. relative molecular mass of salt B; (2 marks)
2 (a) You are provided with solid D. Carry out the following tests and write your observations and inferences in the spaces provided.
(i) Place about one half of solid D in a test-tube and heat it strongly. Test any gases produced with both red and blue litmus papers.
Observations (2 marks)
Inferences (1 mark)
(ii) Place the rest of solid D in a boiling tube. Add about 10cm3 of distilled Water. Shake well.
To a 2cm3 portion of the solution, add about 1cm3 of hydrogen peroxide and shake well.
To the resulting mixture, add aqueous sodium hydroxide dropwise until in excess.
Observations (1 mark)
Inferences(1 mark)
(b) You are provided with solution E. Carry out the following tests and write your observations and inferences in the spaces provided.
Divide solution E into two portions.
(i) To one portion of solution E in a test-tube, add 3 drops of barium nitrate. Retain the mixture for use in test (ii) below.
Observations (1 mark)
Inferences (2 marks)
(ii) To the mixture obtained in (i) above, add about 5 cm3 of 2M nitric (V) acid.
Observations (1 mark)
Inferences (1 mark)
(iii) To portion two of solution E in a test-tube, add 2 drops of acidified potassium dichromate (VI) and warm the mixture.
3 You are provided with liquid F. Carry out the following tests and record your observations and inferences in the spaces provided.
(a) Place five drops of liquid F on a clean dry watch glass and ignite it
Observations(1 mark)
Inferences(1 mark)
(b) Place about 2cm3 of liquid F in a clean dry test tube, add all the sodium hydrogen carbonate provided.
Observations (1 mark)
Inference;(1 mark)
(c) Place about 2cm3 of liquid F in a test-tube, add about 1cm3 of acidified potassium dichromate (VI) and warm the mixture.
Observations (1 mark)
Inferences(1 mark)
