
Chemistry Paper 3 (233/3)
1 You are provided with:
- solution A’ aqueous copper (H) sulphate;
- solid B, iron powder;
- 0.02 M acidified potassium manganate (VII), solution C.You are required to determine the molar heat of displacement of copper by iron.
Procedure I
Using a burette, place 50.0 cm’ of solution A in a 100 ml beaker. Measure the temperature of the solution and record it in table 1 below. Add all of solid B provided at once and start a‘ stop watch. Stir the mixture thoroughly with the thennometer and record the temperature of the mixture after every one minute in the table. Retain the mixture for use in procedure II below.
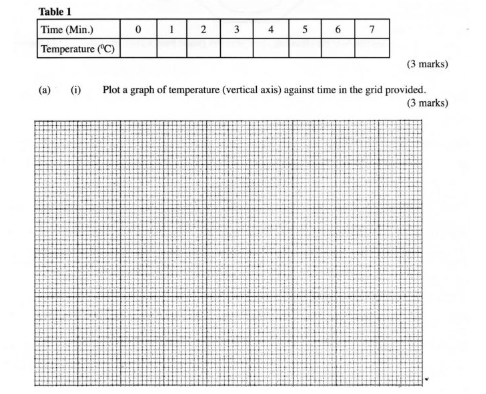
(a) (i) Plot a graph of temperature (vertical axis) against time in the grid provided. (3 marks)
(ii) From the graph, determine the;
(I) highest change in temperature, AT; (1 mark)
(II) time taken for reaction to be completed. (1mark)
(iii) Calculate the heat change for the reaction. (Specific heat capacity of solution is 4.2 Jg” K”; Density of the solution is l gem”). (2 marks)
Procedure II Carefully decant the mixture obtained in procedure I into a 250 ml volumetric flask. Add about 10 cm’ of distilled water to the residue in the 100 ml beaker. Shake well, allow the mixture to settle and carefully decant into the volumetric flask. Immediately, add about 50 cm‘ of 2 M sulphuric (VI) acid to the mixture in the volumetric flask. Add more distilled water to make 250.0 cm‘ of solution. Label this as solution D. Fill a burette with solution C. Using a pipette and a pipette filler, place 25.0 cm’ of solution D into a 250 ml conical flask. Titrate solution D against solution C until the first permanent pink colour is obtained. Record your results in table 2 below. Repeat the titration two more times and complete the table. Retain the remaining solution C for use in question 3.
TABLE II
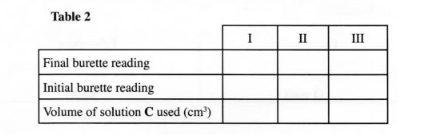
(a) Determine the average volume of solution C used. (1% mark)
(b) Calculate the number of moles of:
(i) aqueous potassium manganate (VII) used; (1 mark)
(ii) iron (II) ions in 25 .0 cm3 of solution D. (1 mole of MnOZ reacts with 5 moles of Fe“). (1 mark
(iii) iron(II) ions in 250 cm‘ of solution D. (1 mark)
(c) Calculate the molar heat of displacement of copper by iron. (2 marks) 2.You are provided with solid E. Carry out the following tests and write your observations and inferences in the spaces provided.
(a) Place all of solid E in a boiling tube. Add about 10 cm’ of distilled water and shake thoroughly. Filter the mixture into another boiling tube. Retain the filtrate for use in test 2
(b) below. Dry the residue using pieces of filter papers.
(i) Transfer about half of the dry residue into a dry test-tube. Heat the residue strongly and test any gas produced using a burning splint.

ii) Place the rest of the residue in a dry test—tube. Add 4 cm’ of 2M hydrochloric acid. Retain the mixture for test (m) below.

iii) To 2 cm-‘ of the solution obtained in (ii) above, add 6 cm-‘ of aqueous ammonia dropwise.

(b) (i) To 2 cm-‘ of the filtrate obtained in (a) above, add about 3 cm3 of aqueous ammonia (Excess).

ii) To 2 cm’ of the filtrate, add about 2 cm’ of 2M hydrochloric acid.

iii) T0 2 cm-‘ of the filtrate, add one or two drops of barium nitrate solution.

3. You are provided with solid G. Carry out the tests in (a) and (b) and write your observations and inferences in the spaces provided. Describe the method used in part (c). (a) Place about one third of solid G on a metallic spatula and bum it in a Bunsen burner flame.

(b) Dissolve all of the remaining solid G in about 10 cm’ of distilled water in a boiling tube. Use the solution for tests
. (i) Place 2 cm-‘ of the solution in a test-tube and add 2 drops of acidified potassium manganate (VII); solution C.

) (ii) To 2 cm‘ of the solution, add all of solid sodium hydrogen carbonate provided.

(c) Determine the pH of the solution obtained in (b) above.

