
Kenya Certificate of Secondary Education
2018 Chemistry paper 1
1. (a) Define a soluble base. (1 mark)
(b) Aqueous solutions of 2M ethanoic acid and 2M nitric(V) acid were tested for electrical conductivity. Which solution is a better conductor of electricity? Explain. (2 marks)
2. (a) Explain why it is not advisable to prepare a sample of carbon(IV) oxide using barium carbonate and dilute sulphuric(VI) acid. (2 marks)
(b) State a method that can be used to collect dry carbon(IV) oxide gas. Give a reason. (l mark)
3. The following are formulae of organic compounds. Use the formulae to answer the questions that follow:
CH3CH CH2OH
CH3 OOH
CH3CH2CH 2CH
CH3CCCH3 (a) Select:
(i) two compounds which when reacted together produce a sweet smelling compound. (1 mark)
(ii) an unsaturated hydrocarbon.(1 mark)
(b) Name the compound selected in (a) (ii).(1 mark)
4. One of the allotropes of sulphur is rhombic sulphur.
(a) Name the other allotrope of sulphur.(1 mark)
(b) Draw a diagram to show the shape of the allotrope named in (a) above.(1 mark)
(c) Write an equation for the reaction between concentrated sulphuric(VI) acid and sulphur.(1 mark)
5. Describe an experiment to show that group one elements react with cold water to form alkaline solutions. (3 marks)
6. (a) State Graham’s law of diffusion. (1 mark)
(b) Explain why a balloon filled with helium gas deflates faster than a balloon of the same size filled with argon gas. (2 marks)
7. 30.0 cm3 of aqueous sodium hydroxide containing 8.0 g per litre of sodium hydroxide were completely neutralised by 0.294 g of a dibasic acid. Determine the relative formula mass of the dibasic acid. (Na = 23.0 ; O = 16.0 ; H 1.0) (3 marks)
8. Study the flow chart in Figure 1 and answer the questions that follow.
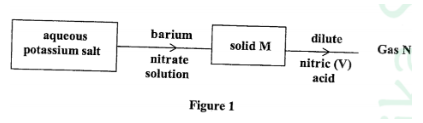
Gas N forms a while suspension with aqueous calcium hydroxide.(1 mark)
(a) Name the anion present in the potassium salt.(1 mark)
(b) Write an ionic equation for the formation of solid M.(1 mark)
(c) Give one use of gas N.(1 mark)
9.An experiment was carried out to determine the presence of substancePQR and S in mixture T.the results obtained are as follows
(a) Name the method of sepration illustrated in figure 2
(ii) a substance wh‹eh is least soluble in t)ie solvent used. 10. Using iron filings, describe an experiment that can be conducted to show that oxygen is present in air. (3 marks)
11. (a) Element U has atomic number 12 while element V has atomic number 16. How do the melting points of their oxides compare? Explain. (3 marks)
12. When ethene gas is compressed at a high temperature, a solid is formed.
(a) Give the name of the solid. (I mark)
(b) Explain why it is not advisable to allow the solid to accumulate in the environment. (2 marks)
13. In the Haber process, nitrogen reacts with hydrogen according to the following equation.
3112(g) + N2(g) 2NH3(g) ; AH = —92 kJ mol**
(a) What would be the effect of adding a catalyst on the position of the equilibrium? (1 mark)
(b) Explain why it is not advisable to use temperatures higher than 773 K in the Haber process. (2 marks)
14. Figure 3 shows a set-up used by a student to prepare dry chlorine gas in the laboratory.
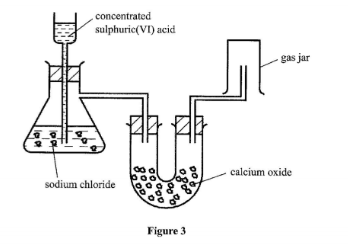
Identify three mistakes in the set-up, and give a reason for each. (3 marks)
15. You are provided with solid potassium hydrogen carbonate. Describe how a solid sample of potassium nitrate can be prepared. (3 marks)
16. Metal X and Y have standard electrode potentials of —0.13 V and —0.76V respectively, T metals were connected to fonn a cell as shown in Figure 4.
(a) Name the part labelled Z.
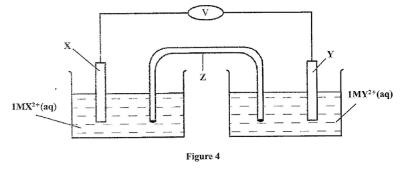
(b) State one function of the part labelled Z.
(c) Calculate the e.m.f. of the cell. (1 mark)
17. Figure 5 represents a grid that is part of the periodic table. Study it and answer the questions that follow. The letters are not the actual symbols of the elements.

(a) Write the electron arrangement of element C. (1 mark)
(b) On the grid provided, show with a tick (I) the position of element D whose atomic number is 18. (1 mark)
(c) Element E is more reactive than A. Explain.
18. (a) Define molar heat of displacement. (1 mark)
(b) The following ionic equation represents the reaction between metal Y and an aqueot solution of Z°+.
Z°+ (aq) + Y(s) Z(s) + Y2 (aq); AH = -ve
Draw an energy level diagram to represent the reaction.
19. (a) Give the symbols of the two charged particles emitted by a radioactive isotope. (2 marks)
(b) An isotope 2 0 Pb disintegrates by emitting two beta particles. Determine the mass number and atomic number of the resulting nuclide.
20. (a) Zinc reacts with hydrochloric acid according to the following equation. Zn(s) + 2HCl (aq) ZnCl2(aq) + H2(g) Identify the reducing agent. Give a reason for the answer. (2 marks)
(b) Iron sheets are dipped in molten zinc to prevent rusting. Name this process. (l mark)
21. Study the set-up in Figure 6 and answer the questions that follow.
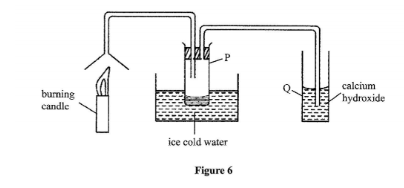
(a) Name the substance that was collected in tube P. (1 mark)
(b) Write an equation for the reaction which occurs in tube Q in the first few minutes of the experiment. (1 mark)
(c) Give a suitable conclusion for the experiment in the set-up. (1 mark)
22. You are provided with the following: thermometer, boiling tube, beaker, Bunsen burner, pure substance X whose boiling point is about 80°C, water and any other apparatus that may be required. Draw a labelled diagram of the set-up that can be used to determine the melting point ofX. (3 marks)
23. Explain why it is important to put off a non-luminous flame immediately after use. (2 marks)
24. (a) Name two ores of iron. (1 mark)
(b) Describe how the amount of iron in a sample of iron(III) oxide can be determined. (2 marks)
25. Explain why a solution of sodium chloride conducts electricity while that of sugar does not. (2 marks)
26. Explain why commercial indicators are preferred to flower extracts as aCid-base indicators. (2 marks)
27. (NH, )2HPO4 is a fertiliser used by farmers to boost their crop production. (a) Calculate the mass of phosphorus in a 20 kg packet of + * 4)2HPO4. (N = 14.0; H — 1.0; P = 31.0; O = 16.0) (2 marks)
(b) State one advantage of this fertilizer, (NH4)2HPO4, over urea (CO(NH 2)2)- (1 mark)
28.Distinguish between emprical and molecular formular of a compond
