
Chemistry Paper 1 (233/1)
1. Increasing the size of the air hole/increase the amount of air/open air holes competely. (1)
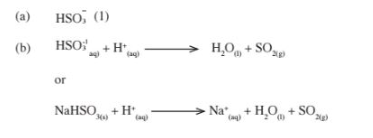
3. (a) – The anhydrous copper (II) Sulphate turns from White to blue. (1)
– A grey solid is formed/droplets of a colourless liquid condense at cool pal“t.(l)
(b) Reducing property.(l)
4. ~ Add soluble carbonate/Add soluble hydroxide. (1)
– Filter out the zinc carbonate/filter the zinc hydroxide. (1)
~ Heat strongly the ZnCO3 to decompose it to form ZnO/Heat strongly the Zn(OH)2 to decompose it to form ZnO. (1)
OR
– Heat to evaporate the water. (1)
– Heat ZnSO4 solid to decompose (1) to form ZnO/yellow solid. (1)
5. (a) delocalised electrons. (1)
(b) ions in the melt. (1)
6.
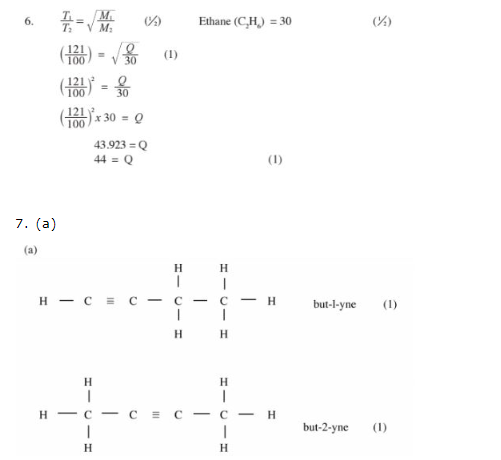
(b) Used in packaging – cushions electronics in boxes/insulation/models/ceiling strips/ crates or binding. (1)
8.
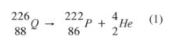
(b) (i) Cobalt 60 is used to detect the activity of the thyroid gland. (1)
(ii) To sterise equipment/treatment of cancer/radio active Na for disorders in blood circulation/Barium meal for ulcers/detect fractures in bones. (l)
9. The molecules of ethanoic acid interact through strong hydrogen bonding (1) forming a dimer while molecules of pentane have weak van der waals forces. (1) NB/ Ethanoic acid has hydrogen bonds while pentane does not have.
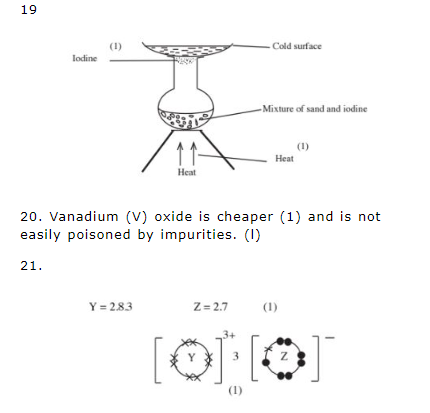
10. (a) Roast ore in air/heat in air. (l)
![]()
(b) – Acid rain that corrodes stone Work on buildings/land gulleys/dust pollution. (1)
– SO2 when breathed in causes bronchitis/chlorosis in plants. (1)
11. Z is SO2/ sulphur (IV) oxide. (1)
M is H2SO3/ sulphuric (IV) acid. (1)
12. A(l)andD(l)
A is acidic it Will neutralise Pb(OH)ZW to form salt and Water, (%)
D is a strong base it Will react with Pb (OH)2W to form a complex ion. (72)
Lead (II) hydroxide is amphoteric.

13.

(b) Some energy is used to ionise the weak acid first before it can neutralise. So not all energy is used in neutralisation. (l)
![]()
The excess ammonia makes solution basic which turns purple with universal indicator. (1)
16. (a) (i) It turned brown /blue/violet/green. (1)
(ii) The Water level rose up the gas jar/occupy space left by reacted O2. (1)
(b) The brown colour would be more since the salt accelerates rusting/rust faster. (1)
17. (a) Rate increases. (1) (b) Temperature increases the kinetic energy (1) of the particles increasing the number of collisions. (1)
18. (a) N (l)
(b) R (1)
(C) M;N2 (1)
22. (a)Condensation of alcohol with higher boiling point so that it runs back to the flask as the alcohol with lower boiling point distills over. (1)
(b) Methanol. (1) It has a lower boiling point due to the size of carbon chain when compared with propanol. (1)
23. (a)Step 1 is neutralisation. (1)
(b) Step II is soda lime/ mixture of NaOH and CaO. (1)
(c) Fuel/making ethene/making hydrogen gas. (1)
24. (a)

26. Natural polymers are biodegradable (1) and are expensive. (1)
Affected by acids/Not easily recyled.
27. (a) Acetone / ethanol / propanone / propanol. (1)
(b) The solvent dissolves the organic compound indicator present in the flowers / it is an organic solvent. (1)
28. (a) It absorbs carbon (IV) oxide present in the air. (1)
(b) Copper /Cuts) (1)
(c) It has rare noble gases which have not been removed / Argon. (1)
29. (a) A radical is a compound formed when elements combine to form ions / free unstable atoms or molecules / a group of free unstable atoms exist in a compound / group of atoms with a common charge. (1)

